Immersion washing of paper is a common process in paper conservation but there are some factors, like pH or conductivity that should be taken into consideration to avoid potential risks of damaging the paper. The key to the effectiveness of paper cleaning may lie with engineering the conductivity of the solutions to the artifact being treated, and controlling the conductivity of the solution during treatment.
Salts naturally occur on paper surfaces and within the paper itself, and are also present in aqueous cleaning solutions used by paper conservators. During aqueous treatments on the paper, the concentration of salts at the water/paper interface undergoes constant changes. These changes are due to the addition of salts to the water, the release of degradation products from the paper into the water, and the transfer of the paper to a fresh bath during cleaning. Conductivity, which is a measure of the number of ions in a solution, is directly influenced by the concentration of salts. Ions are formed when salts naturally dissociate in water.
Traditional paper washing methods that do not consider conductivity can sometimes result in wash baths that are either extremely hypertonic or hypotonic, posing risks of paper damage. It is important to measure and consider the initial conductivity of the paper when preparing washing solutions for two main reasons. Firstly, excessive swelling caused by extreme hypertonic or hypotonic conditions can dislodge fibers and harm the paper's structure. Secondly, maintaining consistent conductivity levels throughout the treatment helps preserve the paper's natural stability and surface characteristics, ensuring that the paper retains its original properties from the beginning to the end of the treatment process.
When paper is immersed in a water-salt solution, both the paper and the solution strive to reach equilibrium, causing ions to move in either direction. This exchange of ions will continue until the conductivities of both the paper and the solution are equal. Monitoring the point at which the conductivity of the solution stops changing during the bath indicates the conclusion of the treatment with that solution. Once equilibrium is reached, there will be no further changes in the paper. Therefore, the paper can then be transferred to a new treatment solution if necessary, or, at some point, the aqueous portion of the treatment can be considered completed.
In the process of cleaning paper, a series of baths are typically conducted to extract degradation products that cause discoloration.

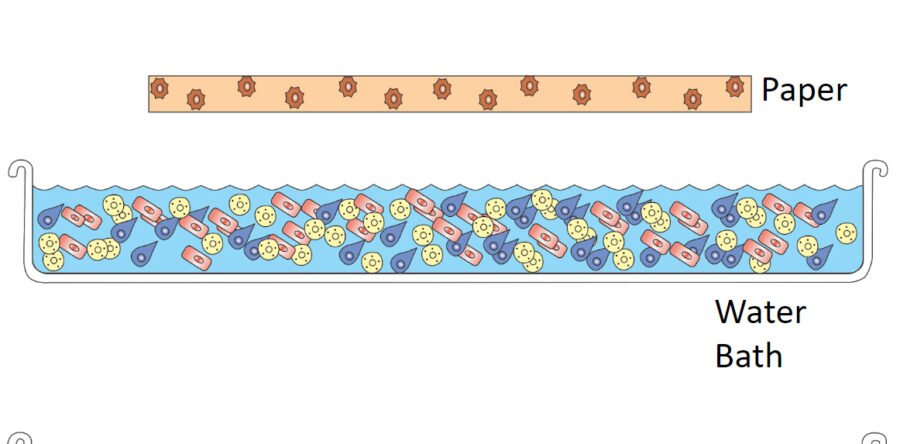
Usually, during the first bath, the paper is exposed to buffered solutions containing salts and potentially chelators that are designed to dislodge degradation products. These additives penetrate the paper through the immersion bath, resulting in an increase in the paper's conductivity. As the paper becomes more conductive, the water's conductivity decreases. Once both the paper and water reach equilibrium, and the conductivity of the water solution stops decreasing, it can be considered that the work done by the solution is completed and the degradation products have been dislodged, solubilized, and converted into ions (image 1). The paper should now be moved to a clearing or rinsing bath.

To effectively remove these dislodged degradation products from the paper, commonly referred to as clearing or rinsing, the paper is immersed in a bath containing inert salts with lower conductivity compared to the original paper. This causes all these substances, including degradation products, salts, and chelators from the initial bath that are now inside the paper, to be released into the rinsing water, as they seek equilibrium. As conductivity naturally moves towards equilibrium, the paper's conductivity decreases while water becomes more conductive. The rinsing process is considered complete when the conductivity level stabilizes and stops changing in the bath (image 2).

After rinsing, the paper's conductivity is typically low. However, the conductivity of the final bath should be even lower. This is because soluble materials will continue to migrate out of the paper due to the tendency of the materials in the bath to reach equilibrium. Nevertheless, the objective is not to deplete the paper, but rather to restore it to its original level. To achieve this, a calcium-based solution with lower conductivity can be used to extract the clearing salts from the paper while replenishing any remaining salts with calcium. Since calcium has a higher affinity for paper compared to other salts, it will remain in the paper as an alkaline reserve, while the clearing salts will be removed into the solution. As the exchange between salts occurs, the water conductivity may slightly increase while the paper conductivity decreases. The bath is considered complete when equilibrium is reached once again.
At this point, the original conductivity level of the paper should have been reached or be very close to it. Therefore, the paper will have regained its original stability, and the degradation products will have been removed and replaced with calcium. An alkaline reserve has been provided to the paper, which will prevent the re-appearance of yellowing on the paper for a while.
Ensuring precise measurement of the initial paper conductivity and tailoring aqueous treatment baths accordingly can help prevent structural damage to the paper. Monitoring conductivity during the treatment process will assist in determining when the action of a bath is completed and the next stage of treatment can commence. Incorporating these kind of procedures as standard practice in paper conservation can provide conservators with safer, more effective, and easily controllable treatment methods.





8 Responses